×
Login Form
×
Registration
Profile Informations
Login Datas
or login
ELISA - Enzyme-linked Immunosorbent Assay
Definition
In the field of immunoassays ELISA (Enzyme Linked Immunosorbent Assay) is used for the detection and, optionally, determination of the concentration of proteins and viruses, or low molecular weight compounds such as hormones, pesticides and toxins in a sample.
The separation of samples (analyte: antibodies or antigens) bound by specific antibodies, that are immobilized on a solid surface such as a polystyrene multiwell plate is characteristic for enzyme immunoassays (EIA).
Principle
Basis for a successful ELISA test is the specific binding of the appropriate antibodies to the epitope, (an amino acid sequence) of the antigen. The antibodies can be both monoclonal-, and polyclonal origin. Monoclonal antibodies derive from so-called hybridoma cells and bind a specific epitope, while polyclonal antibodies recognize different epitopes of an antigen and are a mixture of different antibodies that have been purified from animal serum. For the secondary detection of the analyte polyclonal antibodies are preferably used in indirect ELISA tests. Monoclonal antibodies are used for the primary detection and binding of the antigen due to their specificity for a particular epitope. The concentration of the analyte can be determined by a color reaction and quantitatively evaluated. They replaced the radioimmunoassay by their ease of use and speed.
First, a thin film of a specific antibody or an antigen is coated to a polystyrene multiwell. After an initial blocking step in which still unbound sites on the plate are covered, the detection of the analyte is done by enzyme-conjugated antibodies or antigens. In order to obtain a signal for measuring the bound analyte, a substrate is added, which leads to a color change. For optimal signal exploitation in an ELISA-Test, the washing steps between the processes are particularly important.
ELISA-Types
In addition, ELISA assay formats are distinguished in direct ELISA, indirect ELISA test, sandwich ELISA and competitive or inhibitory ELISA tests.
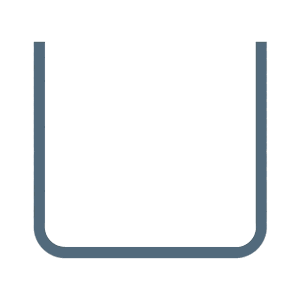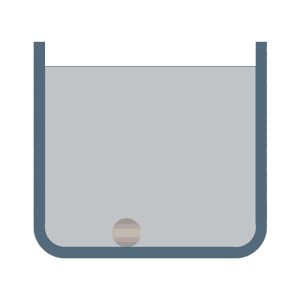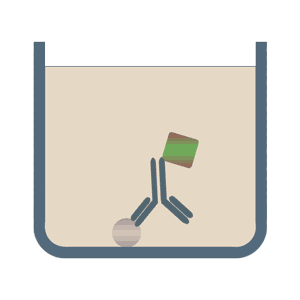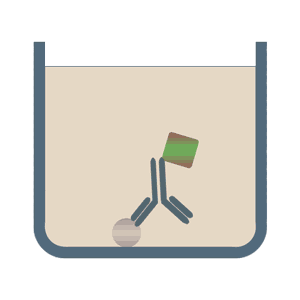
In the case of direct Elisa tests the corresponding antigen or the analyte is detected by an enzyme that is directly conjugated to an antibody. Less common, but still used is exactly the opposite: The specific antibody is attached to the multiwell plate and a labeled antigen is used for the detection. Benefits of the direct ELISA test are its speed and its accuracy, because the number of steps and reagents used are reduced as opposed to the following options.
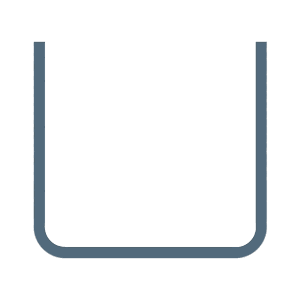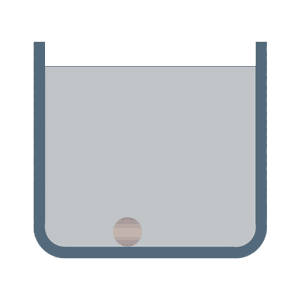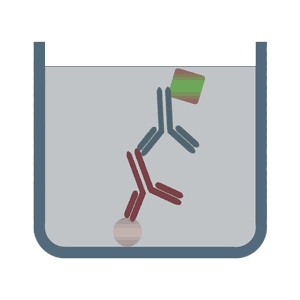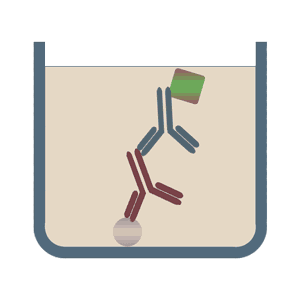
The format of indirect ELISA tests initially uses an unlabeled primary antibody which specifically binds to the antigen of interest. In the second step it is bound by a polyclonal enzyme-conjugated secondary antibody, which is directed against a particular species (for example, anti-mouse). Among the advantages of the indirect ELISA tests is its increased sensitivity, flexibility and lower cost, due to the reduced number of conjugated antibodies.
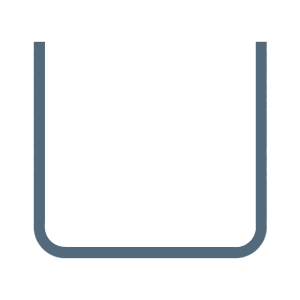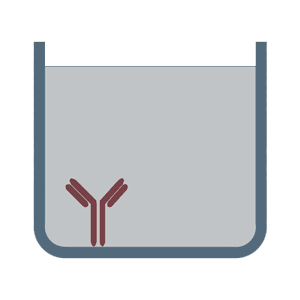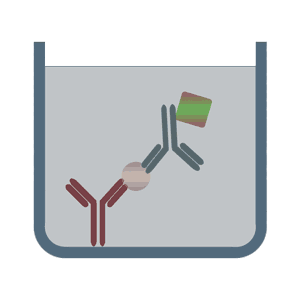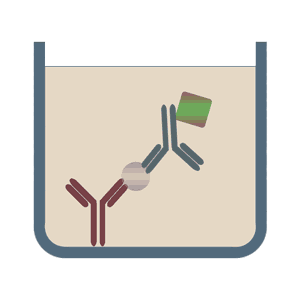
In the sandwich ELISA test two antibodies bind to two different, non-overlapping epitopes on the analyte so that the analyte is bound from both sides like in a sandwich. The polystyrene plate is coated with the first antibody ("capture antibody"). After the sample (including the analyte) is added, the incubation with the secondary antibody ("detection antibody") follows in order to measure the concentration of the analyte. The sandwich ELISA can be distinguished into direct sandwich ELISA, in which the detection antibody is conjugated to an enzyme and the indirect sandwich ELISA, where its detection antibody is unconjugated. The latter method requires an additional conjugated secondary antibody for the measurement. The advantages of the sandwich ELISA test are its flexibility and sensitivity. In addition, it is also possible to specifically use complex sample without prior purification steps.
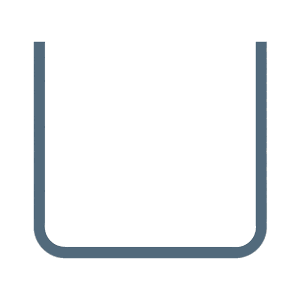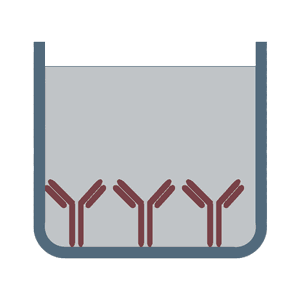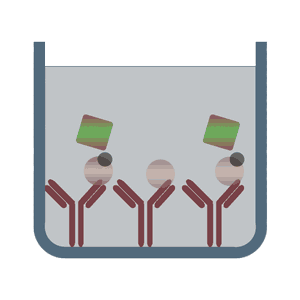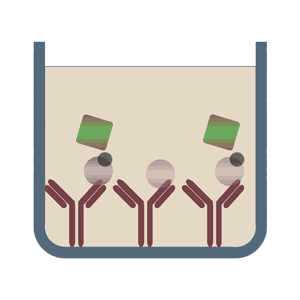
Competitive ELISA test (also referred to as Inhibitive ELISA tests) detect the concentration of the analyte (antibody or antigen) in the sample by influencing or inhibiting the expected signal.
This method is often used when only one antibody to the specific antigen is available, or the analyte is too small to be bound by two different antibodies. The three above-described ELISA tests can build the fundament for the Competitive ELISA test. In the case of a direct ELISA assay format the antigen of the sample would compete with the coated antigen on the multiwell plate for the binding to the detection antibody. In the case of a high concentration of antigen in the sample the signal of the assay would be highly reduced, since the complex of sample antigen and detection antibody would be removed from the assay within the washing steps. Depending on the conjugation of the detection antibody the Competitive ELISA test can be divided into direct and indirect ELISA, too.
In the case of direct detection the alkaline phosphatase (AP) and horseradish peroxidase (HRP), both lead to a colorimetrically measurable color change, are widespread and often used. An alternative to the analysis by a spectrophotometer, fluorescence-labeled antibodies can be chosen too. For indirect detection usually a biotin-conjugated antibody is used. Upon analyte binding, mediated by streptavidin, the conjugated enzyme catalyzes a characteristic color change.
Evaluation of the ELISA
The quality, whether a specific antigen in the sample is present or not, can be examined. Furthermore comparing the signal of the assay to a standard curve, the measured result can be evaluated quantitatively and the present concentration of the antigen can be determined precisely. The graph of ELISA data is built by the application of the optical density versus the logarithm of the concentration, so that the resulting curve has a sigmoidal shape. By comparing results with the linear part of this sigmoidal standard curve, either manually or by the software that came with the ELISA plate reader, the concentration of the sample can be determined.
